Active projects
New synthesis and applications of luminol-UFRJ reagent on hospital infection and crimes against life using portable luminometer apparatus
Description: This work describes the development of a kit to detect occult blood using the luminol reagent, obtained through a new synthesis process without producing waste. The formulation employs enhancing additives luminescence synthesized by cross-coupling reaction of Suzuki- Miyaura being associated with the use of a portable luminometer apparatus. This new technology allows the detection of occult blood in visible light. Enabling the use of luminol in hospitals with the aim of evaluating the disinfection processes in surgical materials, pumps hemodialysis, operating rooms, intensive care units, etc. Contamination with occult blood in the hospital environment has been indicated by recent reports in the literature as a source of cross- transmission of hepatitis C virus in hemodialysis units , with a potential target to enable the presence of other pathogenic microorganisms in various hospital departments, endoscopic devices, multidrug efflux pumps, etc.
Regarding the forensic use employing sprays, the high sensitivity of this formulation of luminol reagent, also allows the use of this analytical tool in the detection of occult blood burned, a biological matrix found at scenes of crime and accidental explosions, beyond the traditional use at crime scenes against human life.
Members: Claudio Cerqueira Lopes - Coordenador / MAYARA A. R. FERREIRA - Member / LETÍCIA G. FERREIRA - Member / R.S.C. Lopes - Member
Members: Claudio Cerqueira Lopes - Coordenador / MAYARA A. R. FERREIRA - Member / LETÍCIA G. FERREIRA - Member / R.S.C. Lopes - Member
Total synthesis of the SIRT1 activator 6,8-dimethoxy-7-(3’,7’-dimethylocta-2’,6’-dienyloxy)benzopyran-2-one (5), a natural product
Description: Chromatin is the primary barrier for gene transcription and its basic structure, the nucleosome, consists of DNA wrapped around proteins, called histones. The chromatin can change its structural conformation according to epigenetic modifications that can occur in the DNA and in the histone tail. Histone deacetylases are a family of proteins responsible for removing the acetyl group from the Ɛ-amino group of lysine residues in histones. They have been classified into four groups according to their homology with yeast proteins. Class I, II and IV are referred to as ‘classical’ HDACs and are Zn²+- dependent whereas class III are referred to as sirtuins and are NAD+-dependent. Sirtuins have been subdivided into 7 other categories and their expression varies according to the tissue. SIRTs 1, 2 and 6 are localized in the nucleus, SIRT2 in the cytosol and SIRTs 3, 4 and 5 are located in the mitochondria. These enzymes constitute of two central domains that combined form a central catalytic histidine residue that is present in almost all organisms, and is proposed to work as an enzymatic core flanked by N- and C- terminals that vary among the sirtuins. SIRT1 can be found in different tissues across the human body. It has been linked to several human conditions such as cancer, cardiovascular disease, insulin sensitivity, energy expenditure, learning and memory and cell survival. In a recent study 6,8-dimethoxy-7-(3’,7’-dimethylocta-2’,6’-dienyloxy)coumarin was compared to Resveratrol, the most potent natural SIRT1 activator, and it was proved to have a similar activity regarding the transcription and activity. Therefore, this work aimed the synthesis of the natural compound through a simple four-steps route consisting in a bromination, displacement of the bromine and methoxylation, cyclization and esterification. The compound was obtained and characterized through ¹H NMR, ¹³C NMR and IR.
Members: Claudio Cerqueira Lopes - Coordenador / ANNA C. SILVA - Member / R.S.C. Lopes - Member.
Members: Claudio Cerqueira Lopes - Coordenador / ANNA C. SILVA - Member / R.S.C. Lopes - Member.
Synthetic approaches for the preparation of antimalarial quindoline via aromatic lithiation reaction
Description: Several research groups have developed alternative syntheses for preparation of the natural product quindoline and its derivatives through synthetic approaches involving the crossed coupling reactions catalyzed by palladium, reductive cyclization mediated by transition metals, photochemical reactions, among others. Such synthetic routes require a great number of steps or additionally, starting reagents that need previous preparation. Indol (3) is an important heterocyclic compound, largely used as an important starting material in the preparation of polyheteroaromatics with relevant biological activity. Frequently, reactions involving abstraction of the hydrogen atom from position C2 in 3,use an organolithium reagent as protective and directing group, linked to the indolic nitrogen, a group which must be easily removed subsequently. In the case of aromatic or heteroaromatic lithiation reactions, some of these groups can be very useful in a range of regioselective functionalization types as orto-metallation directing agents. One of the most elegant methods to promote lithiation with a view to obtaining indol compounds substituted at C2 was developed by Katritzky, using carbon dioxide as the N-protecting group. In this procedure, the N-protecting group is inserted in situ and removed at the end of the reaction, eliminating deprotecting steps. We describe two approaches for the preparation of natural alkaloid quindoline (1) from indol (3) through a very direct synthetic strategy. Several successive heteroaromatic lithiation reaction steps were performed in the same medium producing bis benzylic alcohol (7) in excellent yield. The alcohol was submitted to catalytic reduction, undergoing simultaneous cyclization and aromatization, yielding quindoline (1) in 55% overall yield.
Members: Claudio Cerqueira Lopes - Coordenador / DAYSE S. BASTOS - Member / R.S.C. Lopes - Member.
Members: Claudio Cerqueira Lopes - Coordenador / DAYSE S. BASTOS - Member / R.S.C. Lopes - Member.
A new approach of analysis to evaluate the quality of captopril tablets using HPLC
Description: This work describes the analytical HPLC method for the quantification of captopril and its degradation product, the captopril disulfide in captopril tablet 25 mg. For the development of analytical method, a comparative study of sample solutions stability of commercial batches of captopril tablets in a variety of solvents was carried out within a period of 24 hours, in order to evaluate in that diluent the sample solutions would present better stability. The aim of this study was to investigate analytical alternatives so that is minimized the oxidative degradation of captopril during the time of analysis, compared with the method of analysis described in the Brazilian Pharmacopoeia 5th edition and U.S. Pharmacopeia (USP 36). In analytical terms proposed in the official compendium - Brazilian Pharmacopoeia 5th edition and U.S. Pharmacopoeia 36 - the analysis method for the determination of captopril, as for the quantification of its degradation product of both the disulfide of captopril demonstrated to be inadequate. In terms of stability of the solution within 24 hours which can be seen the analytical results, was observed a significant decreasing of captopril with a significant increasing of the amount of captopril disulfide in the tablets when samples were analyzed during the period time of analysis proposed. Then we tried to keep the methodology of HPLC analysis, because it is a fast, accurate and stable method, changing only the diluent employed in the dilution of samples and standards in order to find a solvent that promotes captopril stability in solution for a period of at least 24 hours. At the end of the study, it was found that the solvent methanol among the tested solvents it demonstrated a great stability of sample solutions of captopril tablets with different validity times.
Members: Claudio Cerqueira Lopes - Coordenador / JOVÂNIA F. R. PAIVA - Member / ANDRÉ L. M. ALBERT - Member / R.S.C. Lopes - Member.
Members: Claudio Cerqueira Lopes - Coordenador / JOVÂNIA F. R. PAIVA - Member / ANDRÉ L. M. ALBERT - Member / R.S.C. Lopes - Member.
Synthesis of 1-O-palmityl-sn-glycerol and its aryl boronates derivatives with potential antifouling activity
Description: The naturally occurring 1-O-alkyl-sn-glycerols and their methoxylated congeners, 1-O-(2-methoxyalkyl)-sn-glycerols, are biologically active compounds, ubiquitously found in nature as diacyl glyceryl ether lipids and phosphoether lipids. The unsaponifiable fractions from cartilaginous fish, isolated and recorded the chemical properties of the 1-O-alkyl-sn-glycerols comprising stearyl (C18:0), palmityl (C16:0), and oleyl (C18:1) alkyl chains and gave them the trivial names batyl, chimyl, and selachyl alcohols in accordance with the chondrichthyans they were isolated from i.e. Batoidea (rays), Chimearas (ratfish), and Selachii (sharks), respectively. Further studies demonstrated that in marine oils 1-O-alkyl-snglycerols were present as fatty acid diesters, also called diacyl glyceryl ethers, although, they were not isolated as such until 1960. The discovery of plasmalogens and their analogues plasmanyl phospholipids demonstrated that 1-O-alkyl-sn-glycerols were not only restricted to marine animal species, but they were in fact ubiquitous constituents of animal cell membranes. Furthermore, a platelet-activating factor (PAF), 1-O-alkyl-2-acetyl-sn-glyero-3-phosphocholine was characterized as being a group of highly versatile signal mediators found in very low concentration endogenously released by mammal cells involved in inflammatory reaction and reproductive functions. The naturally occurring 1-O-alkyl-sn-glycerols, also referred to as alkylglycerols, are mainly found as 1-O-alkyl-2,3-diacylsn-glycerols and 1-O-alkyl-2-acyl-sn-glycero-3-phospholipids. We are developing a synthetic route for the preparation of natural 1-O-palmityl-sn-glycerol and its aryl boronates derivatives which will be tested on biofouling control for use in paints to ship and off shore units of petroleum prospect. The epychloridrine synthetized from glycerol was submitted on the presence of cetyl alcohol, aqueous solution of sodium hydroxide and tetra-N-butyl ammonium hydrogensulphate furnishing 1-(hexadecyloxymethylene)oxirane in 80% yield after purification through flash chromatography. We will present several attempts to obtain this important compound and the influence of other types of surfactants on this type of reaction as well as the preparation of 1-O-palmityl-sn-glycerol.
Members: Claudio Cerqueira Lopes - Coordenador / LUCIANA G. MONTEIRO - Member / WILLIAM R. BATISTA - Member / R.S.C. Lopes - Member.
Members: Claudio Cerqueira Lopes - Coordenador / LUCIANA G. MONTEIRO - Member / WILLIAM R. BATISTA - Member / R.S.C. Lopes - Member.
SCIENCE WITHOUT BORDERS COLLABORATIONS
Colaboração com o Professor Arasu Ganesan
Department of Medicinal Chemistry, University of East Anglia, Norwich, Norfolk, England
Description: The collaboration will be based around the area of organic synthesis, chemical biology and medicinal chemistry. It will provide excellent training for students and postdoctoral associates from Brazil and the opportunity for them to participate in cutting-edge research in a leading UK university and improve their English language skills. Among the specific benefits to researchers will be.
a) Implementation of new strategies for target-oriented organic synthesis and application of procedures using modern reagents;Members: Claudio Cerqueira Lopes / Arasu Ganesan / R.S.C. Lopes.
b) Methods for library design based on physicochemical filtering and structure-based computational docking against specific targets;
c) Synthetic routes to heterocyclic scaffolds with potential against neglected and tropical diseases;
d) Access to instrumentation for organic synthesis such as solid-phase synthesizers, parallel synthesis equipment and microwave heating;
e) Access to analytical tools such as high-field NMR (800 MHz, 500 MHz, 400 MHz, both solution and solid-sate), coupled MS techniques, sequencing facilities;
f) Participation in undergraduate and postgraduate classes and special courses on advanced topics;
g) Participation in seminar series at UEA with external speakers;
h) Attendance at scientific conferences in Europe and participation by poster and oral presentation;
Project: Wedelolactone
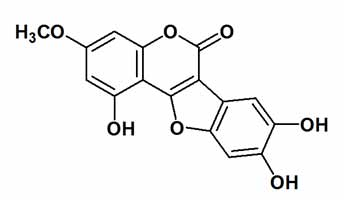
Description: Extracts of the plants Eclipta alba, Eclipta prostrata and Wedelia chinensis have a long history of use in traditional Chinese and ayurvedic medicine for the treatment of diseases of the central nervous system, liver, and eyes and the prevention of hair loss. The extracts are known for their liver detoxifying properties and are used as venom against scorpion and snake bite in China and Brazil. The active principle in these extracts is the aromatic natural product wedelolactone. Wedelolactone inhibits the NF-KB pathway of inflammation by directly inhibiting the IKK α and β kinase activity and is widely used as a biological probe. Nevertheless, the systematic study of the properties of wedelolactone is hampered by the unavailability of suitable analogues for structure-activity relationship (SAR) studies.
Our objective is to accomplish a short and scalable route to this highly biologically active molecule. Previous total syntheses of wedelolactone have taken nearly 20 steps. We have designed a significantly shorter route that will be under 10 steps. Our synthesis is modular and will not only provide access to the natural product but also a variety of unnatural analogues for biological investigation. This work is a joint collaboration between the research groups of Professor Claudio and Rosangela Lopes at UFRJ, Brazil and Professor A. Ganesan at UEA, UK.